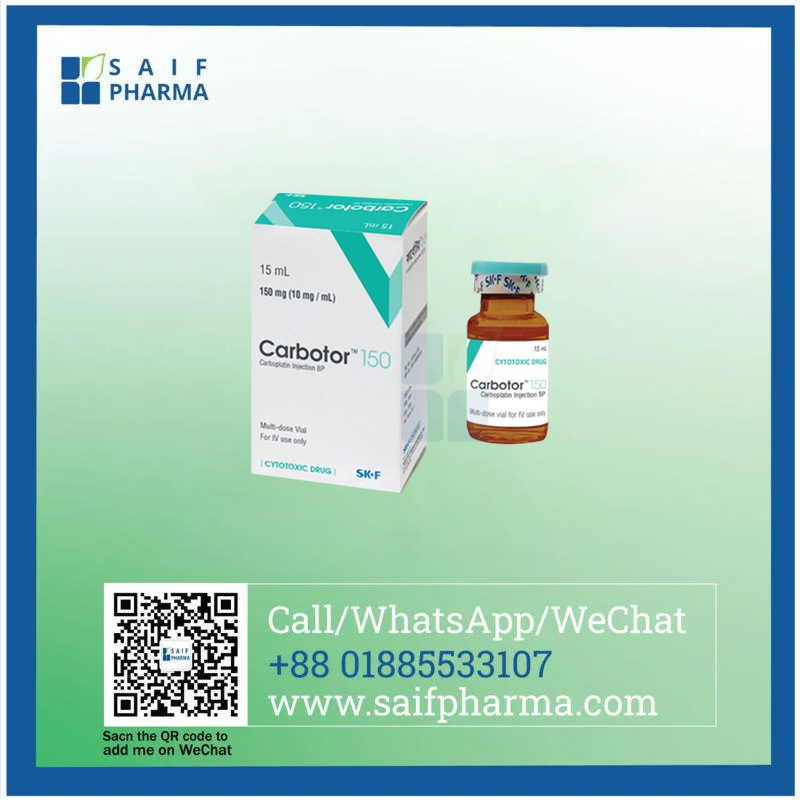
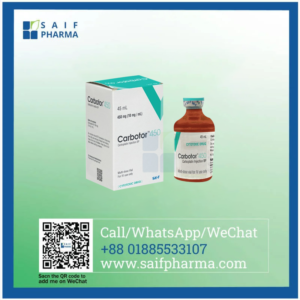
Introduction to Carbotor 150 mg (Carboplatin) Injection
Carbotor 150 mg, containing the active ingredient Carboplatin, is a platinum-based chemotherapy drug widely used in the treatment of ovarian, lung, head and neck, and other cancers. Manufactured by Eskayef Pharmaceuticals Ltd. and supplied by Saif Pharma, this injection is a cornerstone of modern oncology regimens, offering precision, efficacy, and safety in cancer management.
Key Indications & Therapeutic Benefits
- Ovarian Cancer: First-line and recurrent treatment.
- Lung Cancer: Used in non-small cell lung cancer (NSCLC).
- Other Cancers: Effective for head and neck, bladder, and germ cell tumors.
- Mechanism of Action: Carboplatin cross-links DNA strands, inhibiting cancer cell replication and inducing apoptosis.
Pharmacology & Pharmacokinetics
- Chemical Class: Platinum coordination compound (analog of cisplatin).
- Absorption: Administered intravenously for direct systemic delivery.
- Distribution: Binds to plasma proteins and distributes widely, including into tumors.
- Metabolism: Minimal hepatic metabolism; primarily excreted renally (60-90% within 24 hours).
- Half-Life: Approximately 2-6 hours, requiring calculated dosing based on renal function.
Recommended Dosage & Administration
- Dosing Formula: Calvert Formula (AUC-based): Dose (mg) = Target AUC × (GFR + 25).
- Standard Regimens:
- Ovarian Cancer: AUC 5-6 every 3 weeks.
- Lung Cancer: AUC 5-6 in combination with paclitaxel.
- Administration:
- Administered as an IV infusion over 15-60 minutes.
- Pre-hydration not required (unlike cisplatin).
- Dose Adjustments:
- Renal Impairment: Reduce dose for CrCl <60 mL/min.
- Myelosuppression: Monitor blood counts; delay dose if platelets <100,000/mm³ or neutrophils <2,000/mm³.
Drug Interactions & Precautions
- Nephrotoxic Drugs (e.g., NSAIDs): May exacerbate kidney damage.
- Myelosuppressive Agents: Increased risk of bone marrow suppression.
- Live Vaccines: Avoid during treatment due to immunosuppression.
- Pregnancy & Lactation:
- Contraindicated – may cause fetal harm.
- Advise contraception during and for 6 months post-treatment.
- Avoid breastfeeding.
Contraindications & Safety Warnings
- Hypersensitivity: Avoid in patients with platinum compound allergies.
- Severe Bone Marrow Suppression: Baseline monitoring of CBC, renal function, and electrolytes required.
- Renal Dysfunction: Dose adjustment mandatory for CrCl <60 mL/min.
- Peripheral Neuropathy: Monitor for numbness, tingling, or pain.
Common Side Effects
- Hematologic:
- Thrombocytopenia (low platelets) – 25-35%.
- Neutropenia (low neutrophils) – 15-20%.
- Anemia (low RBCs) – 10-15%.
- Gastrointestinal: Nausea (50%), vomiting (30%), diarrhea (10%).
- Neurologic: Peripheral neuropathy (10%), ototoxicity (rare).
- Other: Fatigue, alopecia, elevated liver enzymes.
Storage & Handling
- Storage: Store at 20-25°C; protect from light.
- Reconstitution: Use sterile water or saline; stable for 8 hours at room temperature.
- Disposal: Follow hazardous waste protocols for cytotoxic drugs.
Why Choose Carbotor from Saif Pharma?
Saif Pharma is a globally recognized supplier of oncology medications, committed to delivering high-quality, affordable chemotherapy drugs to 60+ countries, including the USA, Canada, UAE, and India. Our partnership with Eskayef Pharmaceuticals ensures adherence to international manufacturing standards, making Carbotor 150 mg a trusted choice for oncologists and patients worldwide.
Manufacturer & Supplier Credentials
- Manufacturer: Eskayef Pharmaceuticals Ltd. – A flagship enterprise of Bangladesh’s Transcom Group, producing WHO-GMP-certified medicines since 1990.
- Supplier: Saif Pharma – A leader in oncology distribution since 2014, dedicated to reducing global cancer burden through accessible therapies.
Clinical Efficacy & Safety Profile
Carbotor (Carboplatin) is preferred over cisplatin due to its reduced nephrotoxicity and ototoxicity, making it safer for patients with pre-existing kidney issues. Clinical trials demonstrate response rates of 15-30% in ovarian cancer and improved survival in lung cancer when combined with taxanes.
Patient Support & Counseling
- Pre-Medication: Antiemetics (e.g., ondansetron) recommended to manage nausea.
- Hydration: Maintain adequate fluid intake to support renal excretion.
- Monitoring: Regular CBC, renal function tests, and audiometric exams (if symptoms arise).
Conclusion
Carbotor 150 mg (Carboplatin) Injection is a vital chemotherapeutic agent in the fight against cancer, offering balanced efficacy and tolerability. For authentic, cost-effective access to this life-saving medication, trust Saif Pharma – your global partner in oncology care.