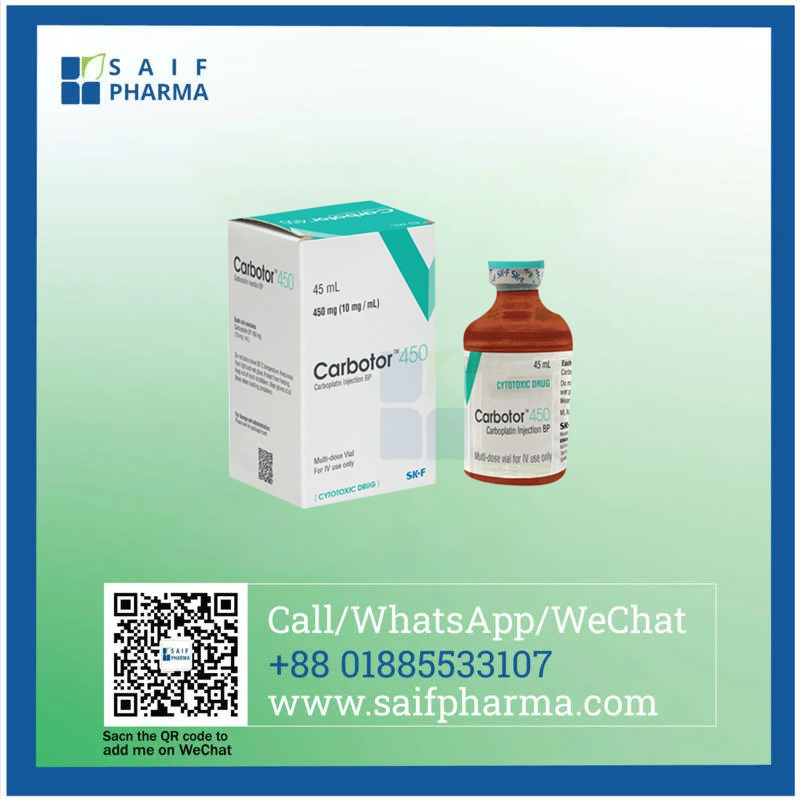
Introduction to Carbotor 450 mg (Carboplatin) Injection
Carbotor 450 mg, formulated with Carboplatin, is a high-dose platinum-based chemotherapy injection designed for advanced cancer treatment. Manufactured by Eskayef Pharmaceuticals Ltd. and supplied globally by Saif Pharma, this injection is pivotal in treating ovarian cancer, lung cancer, and other malignancies, offering a balanced efficacy-toxicity profile compared to cisplatin.
Key Indications & Therapeutic Benefits
- Ovarian Cancer: First-line therapy and recurrent disease management.
- Lung Cancer: Effective for non-small cell lung cancer (NSCLC) in combination regimens.
- Other Cancers: Used in head and neck, bladder, testicular, and germ cell cancers.
- Mechanism of Action: Carboplatin cross-links DNA, disrupting replication and triggering apoptosis in cancer cells.
Pharmacology & Pharmacokinetics
- Chemical Class: Platinum-based alkylating agent (analog of cisplatin).
- Absorption: Administered intravenously (IV), ensuring rapid systemic distribution.
- Distribution: Binds extensively to plasma proteins; penetrates tumor tissues effectively.
- Metabolism: Minimal hepatic metabolism; 90% excreted renally within 24 hours.
- Half-Life: 2-6 hours, necessitating renal function-based dosing adjustments.
Recommended Dosage & Administration
- Dosing Formula: Calvert Formula guides dosing: Dose (mg) = Target AUC × (GFR + 25).
- Typical Regimens:
- Ovarian Cancer: AUC 5-6 every 3-4 weeks.
- Lung Cancer: AUC 5-6 combined with taxanes (e.g., paclitaxel).
- Typical Regimens:
- Administration Guidelines:
- IV Infusion: Administered over 15-60 minutes; no pre-hydration required.
- Dose Adjustments:
- Renal Impairment: Reduce dose for CrCl <60 mL/min (consult prescribing guidelines).
- Myelosuppression: Delay if platelets <100,000/mm³ or neutrophils <2,000/mm³.
- 450 mg Strength: Enables flexible dosing for patients requiring higher AUC targets or reduced infusion frequency.
Drug Interactions & Precautions
- Nephrotoxic Agents (e.g., NSAIDs): Risk of compounded kidney damage; monitor renal function.
- Myelosuppressive Drugs: Increased risk of severe anemia, neutropenia, or thrombocytopenia.
- Live Vaccines: Avoid during treatment due to immunosuppression.
- Pregnancy & Lactation:
- Contraindicated – potential teratogenicity.
- Advise contraception during and 6 months post-treatment.
- Discontinue breastfeeding.
Contraindications & Safety Warnings
- Hypersensitivity: Avoid in patients with platinum compound allergies.
- Severe Renal Dysfunction: Dose adjustments mandatory (CrCl <60 mL/min).
- Bone Marrow Suppression: Baseline and regular CBC monitoring required.
- Peripheral Neuropathy: Monitor for sensory deficits or pain.
Common Side Effects
- Hematologic:
- Thrombocytopenia (30-40% incidence).
- Neutropenia (20-25%).
- Anemia (15-20%).
- Gastrointestinal: Nausea (50-60%), vomiting (30-40%), mild diarrhea.
- Neurologic: Peripheral neuropathy (10-15%), rare ototoxicity.
- Other: Fatigue, alopecia, transient liver enzyme elevation.
Storage & Handling
- Storage: Store at 20-25°C; protect from light.
- Reconstitution: Use sterile water/saline; stable for 8 hours post-mixing.
- Disposal: Follow cytotoxic waste protocols to ensure safety.
Why Choose Carbotor 450 mg from Saif Pharma?
Saif Pharma is a globally trusted supplier of oncology medications, delivering high-quality, cost-effective chemotherapy drugs to 60+ countries, including the USA, Canada, UAE, and India. Partnering with Eskayef Pharmaceuticals—a WHO-GMP-certified manufacturer—ensures rigorous quality control, making Carbotor 450 mg a reliable choice for oncologists and patients.
Manufacturer & Supplier Credentials
- Manufacturer: Eskayef Pharmaceuticals Ltd. – A pioneer under Bangladesh’s Transcom Group, producing globally compliant medicines since 1990.
- Supplier: Saif Pharma – Specializing in oncology since 2014, committed to reducing cancer mortality through accessible therapies.
Clinical Advantages of Carbotor 450 mg
- Reduced Toxicity: Lower nephrotoxicity and ototoxicity vs. cisplatin.
- Dosing Flexibility: Higher strength (450 mg) allows tailored regimens for improved patient compliance.
- Proven Efficacy: Clinical trials show response rates up to 35% in ovarian cancer and enhanced survival in lung cancer when combined with taxanes.
Patient Management Tips
- Pre-Medication: Use 5-HT3 antagonists (e.g., ondansetron) to mitigate nausea/vomiting.
- Hydration: Encourage fluid intake to support kidney function.
- Monitoring: Regular blood tests (CBC, renal function) and neurologic assessments.
Conclusion
Carbotor 450 mg (Carboplatin) Injection is a cornerstone of modern chemotherapy, offering precision and safety in cancer care. For authentic, affordable access to this life-saving medication, trust Saif Pharma—your global partner in oncology excellence.